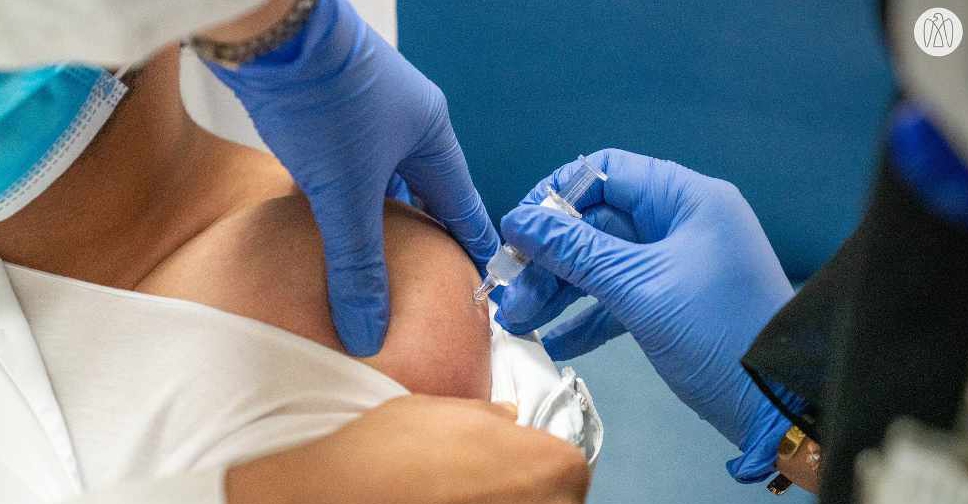
The UAE is taking all possible measures to ensure the safety and efficacy of the COVID-19 vaccine trial which is currently underway in the country.
Volunteers signing up for the trials will be monitored for a period of at least six months with daily follow-ups as well as testing for the virus at regular intervals.
Ashish Koshy is the CEO of G42 Healthcare, which is conducting the third phase of clinical trials in cooperation with China's Sinopharm.
He told ARN's Dubai Eye on One how the entire process works.
Koshy added that if the tests are successful, the vaccine could be made available to UAE residents by early 2021.
Five thousand volunteers signed up to test the inactivated vaccine within 24 hours of authorities announcing the registration process on Thursday.
UAE nationals and residents between the ages of 18 and 60 are eligible to sign up for the study, provided they pass the medical checks.
.@mohapuae and @DoHSocial are coordinating to open registration for nationals and residents across the UAE who wish to volunteer for Phase III clinical trials of inactivated vaccine, and to specify locations for receiving volunteers. More details to be published soon. #4Humanity pic.twitter.com/ejHV9Ypb0w
— مكتب أبوظبي الإعلامي (@ADMediaOffice) July 19, 2020
.@DoHSocial announces the registration of 5000 volunteers for the third phase of clinical trials for inactivated COVID-19 vaccine in #AbuDhabi during the first 24 hours of activating the registration site https://t.co/Jj8zC4KAzV pic.twitter.com/MwUqgjcKOR
— مكتب أبوظبي الإعلامي (@ADMediaOffice) July 17, 2020

 UAE to begin lifting drone-use restrictions
UAE to begin lifting drone-use restrictions
 Dubai Police warn residents of scam calls
Dubai Police warn residents of scam calls
 UAE President welcomes Indonesian counterpart
UAE President welcomes Indonesian counterpart
 Indonesian President arrives in UAE on state visit
Indonesian President arrives in UAE on state visit
